Technical dismantling of lithium battery
Lithium battery can be said to be the basis for all battery applications. Many innovative batteries are based on lithium batteries. This time, we will share a technical guide about lithium batteries so that everyone can understand everything from the electrochemical process in lithium batteries to complex voltage regulation so that it can be easier in daily use, research, and development.
Lithium battery analysis components
Lithium batteries mainly comprise two large parts: the battery cell and the protective plate PCM. The battery cell is equivalent to the heart of the lithium battery, and the management system is equal to the brain of the lithium battery. The battery core mainly comprises positive electrode material, harmful electrode material, electrolyte, diaphragm, and casing. In contrast, the protection board primarily comprises a protection chip, MOS tube, resistor, capacitor, and PCB board. The cathode, usually composed of lithium cobalt oxide, interacts with the anode, generally made of graphite and separated by a microporous polymer separator soaked in an electrolyte solution. As shown below:
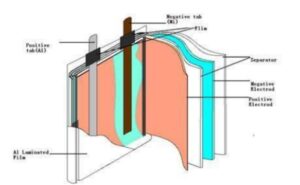
Interpret the electrochemical reactions of lithium batteries.
The magic of lithium batteries unfolds through a complex series of electrochemical reactions in which lithium ions shuttle between the anode and cathode during charge and discharge cycles. When lithium ions move from the anode to the cathode, the discharge releases energy, producing electricity. Charging reverses this process. The choice of cathode material, such as lithium cobalt oxide or iron phosphate, can significantly impact performance metrics, allowing engineers to fine-tune and optimize battery chemistry.
During discharge (when the battery delivers power), lithium ions move from the anode (usually made of graphite) to the positive electrode (cathode). The overall reaction of a discharge is the movement of lithium ions from anode to cathode, producing an electric current, which can be used to power electronic devices. During charging, lithium ions move from the cathode back to the anode when an external potential is applied to the battery. This process of the overall charge reaction effectively reverses the discharge reaction, allowing the battery to store electrical energy for later use. As shown below:
Voltage, capacity and energy density of lithium battery
Key metrics such as voltage, capacity, and energy density measure lithium battery performance and physical components. Measuring in volts, voltage represents the potential difference between the anode and cathode. Power is measured in ampere hours (Ah) and represents the total amount of electricity a battery can store. Energy density evaluates how much energy a battery can store per unit volume or weight. Technological advancements continue to push these indicators to new heights, improving the efficiency and applicability of lithium batteries in various devices. Engineers and researchers continue to explore innovative materials and designs to improve these metrics, ensuring that lithium batteries remain at the forefront of energy storage solutions. As shown below:
Dealing with high temperatures and safety
The efficiency of lithium batteries faces challenges, especially heat generation and safety issues. Exothermic reactions during charge and discharge cycles raise temperatures, creating a risk of thermal runaway. If the temperature decreases, the reaction rate of the electrode also decreases. Assuming the battery voltage remains constant and the discharge current decreases, the battery’s power output will also decrease. If the temperature rises, the opposite is true: the battery output power will increase, and the temperature will also affect the transmission speed of the electrolyte. As the temperature rises, it accelerates, decreases the transmission temperature, slows the transmission, and affects the performance of battery charge and discharge.
Advanced thermal management systems and safety mechanisms such as built-in circuitry and protective casing are integral to mitigating these challenges. Engineers use cutting-edge materials and design techniques to ensure lithium batteries maintain strong performance and are safe for various applications. This multifaceted approach involves a careful balance between performance optimization and stringent safety standards, reflecting the evolving nature of lithium battery technology.

Research on solid-state lithium batteries
A critical frontier in lithium battery research revolves around exploring solid-state batteries, which aim to replace traditional liquid electrolytes with solid alternatives. Solid-state batteries improve safety by eliminating leakage and thermal runaway risks. Additionally, they pave the way for increased energy density and longer cycle life. Engineers delve into the complexities of solid electrolyte materials to address issues related to conductivity and manufacturability. The successful realization of commercially viable solid-state lithium batteries heralds a new era of energy storage, where safety and performance merge in the solid state.
In the end
As the technology evolves, so does the potential of lithium batteries, shaping the future of portable energy storage and sustainable power solutions. Hopefully, what we’ve shared in this guide will give you the insights to prepare you for pioneering advances that will further advance lithium battery technology and solidify its role as a driver toward a more sustainable and efficient energy future.

